Pharmacological Classification
Class: Biguanide
ATC Code: A10BA02
Mechanism of Action (MOA)
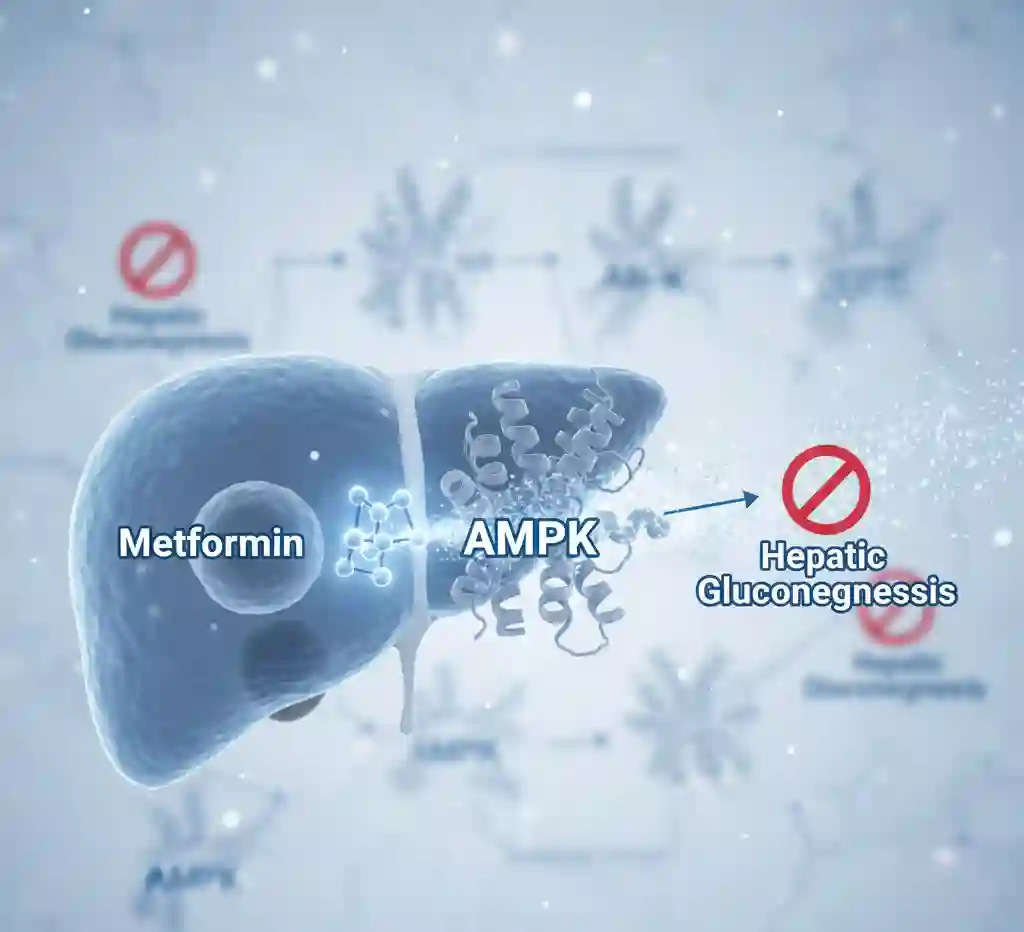
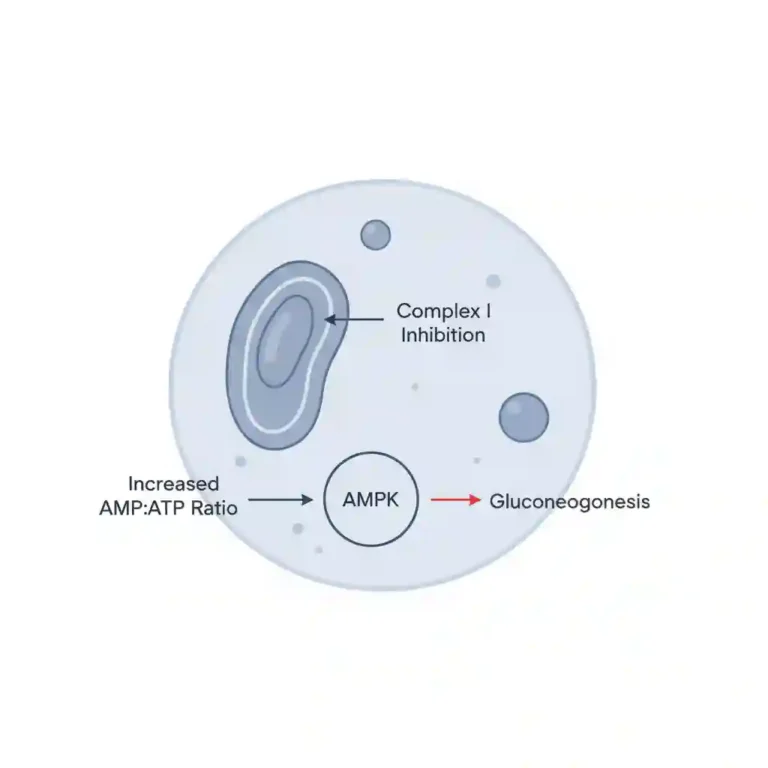
Metformin’s primary antihyperglycemic effect is achieved through the activation of AMP-activated protein kinase (AMPK), a key enzyme in cellular energy homeostasis. This activation leads to several downstream effects. In the liver, it inhibits hepatic gluconeogenesis and glycogenolysis, effectively reducing the endogenous production of glucose. In peripheral tissues, particularly skeletal muscle, metformin enhances insulin sensitivity. It does this by increasing glucose uptake and utilization, primarily by promoting the translocation of GLUT4 glucose transporters to the cell membrane. Additionally, it may modestly decrease the intestinal absorption of glucose.
Pharmacokinetics (PK Profile)
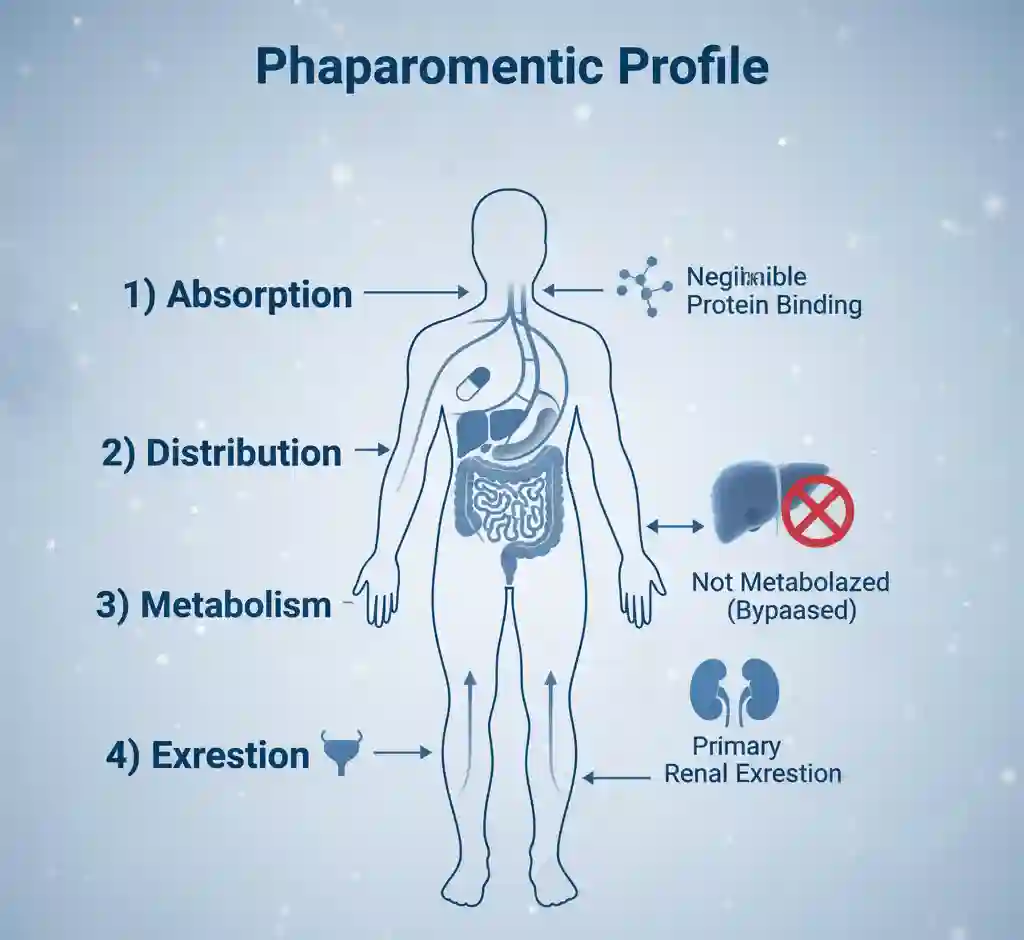
- Absorption: The absolute bioavailability of metformin hydrochloride is approximately 50-60% under fasting conditions. Absorption is incomplete and saturable.
- Distribution: It exhibits negligible binding to plasma proteins and partitions into erythrocytes. The volume of distribution is large.
- Metabolism: Metformin is not metabolized in the liver or any other tissues. It is excreted unchanged.
- Excretion: Excretion is accomplished via active tubular secretion in the kidneys. The plasma elimination half-life is approximately 6.2 hours.
Clinical Indications (FDA Labeling)
Metformin is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus. Common trade names include Glucophage, Fortamet, and Glumetza. It is also frequently used in an off-label capacity for the management of Polycystic Ovary Syndrome (PCOS).
Contraindications & Black Box Warnings
The U.S. FDA has issued a Black Box Warning regarding Lactic Acidosis. This rare but serious metabolic complication can occur due to metformin accumulation. The risk is increased in patients with renal impairment, concomitant use of certain drugs, and in hypoxic states.
Contraindications include:
- Severe renal impairment (e.g., eGFR below 30 mL/min/1.73 m²).
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis.
- Known hypersensitivity to metformin hydrochloride.