Executive Summary
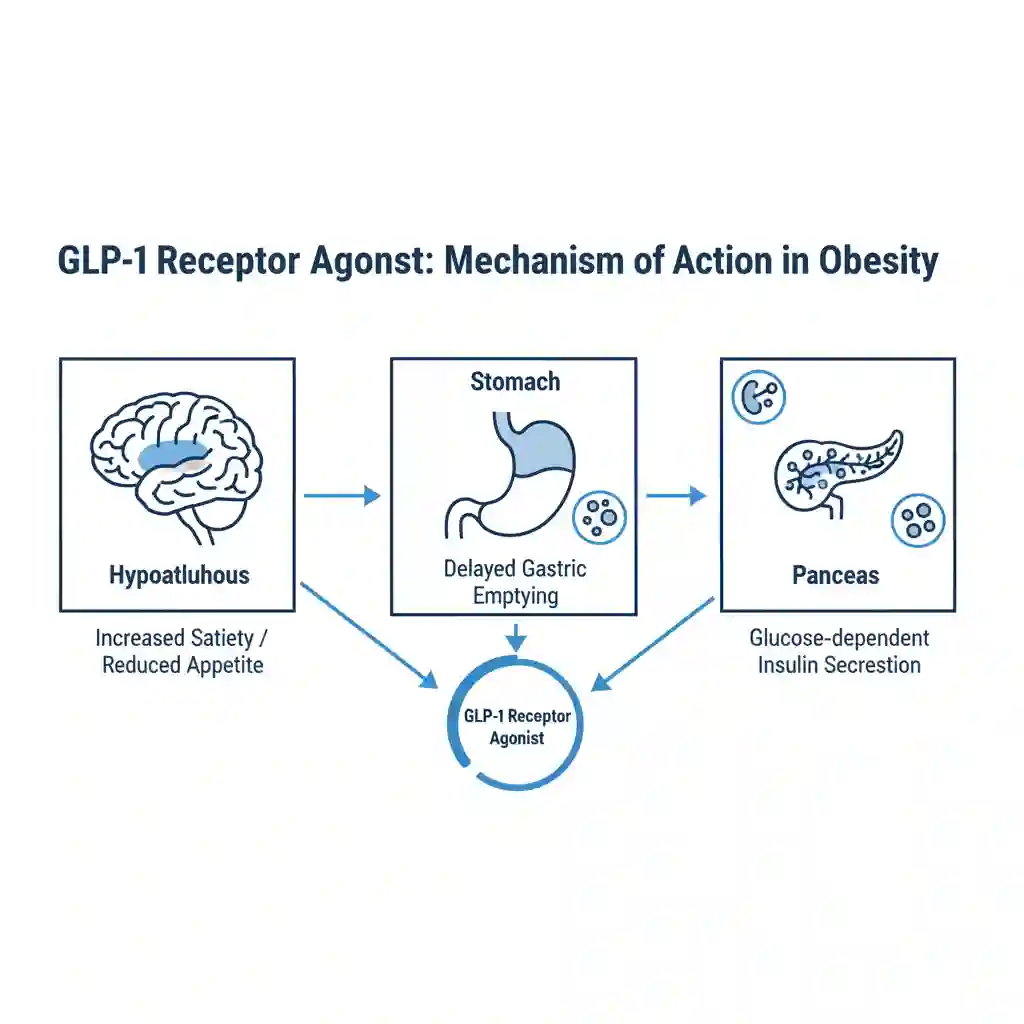
The landscape of obesity management has undergone a paradigm shift with the introduction and FDA approval of potent Glucagon-like Peptide-1 (GLP-1) receptor agonists (e.g., semaglutide) and dual GIP/GLP-1 agonists (e.g., tirzepatide). Originally developed for Type 2 Diabetes management, these agents mimic the action of endogenous incretin hormones. Recent high-impact research demonstrates their efficacy in inducing significant, sustained weight loss through central appetite suppression and delayed gastric emptying. This trend marks a transition from viewing obesity solely as a behavioral issue to treating it as a chronic, relapsing neuroendocrine disease.
Key Data Points
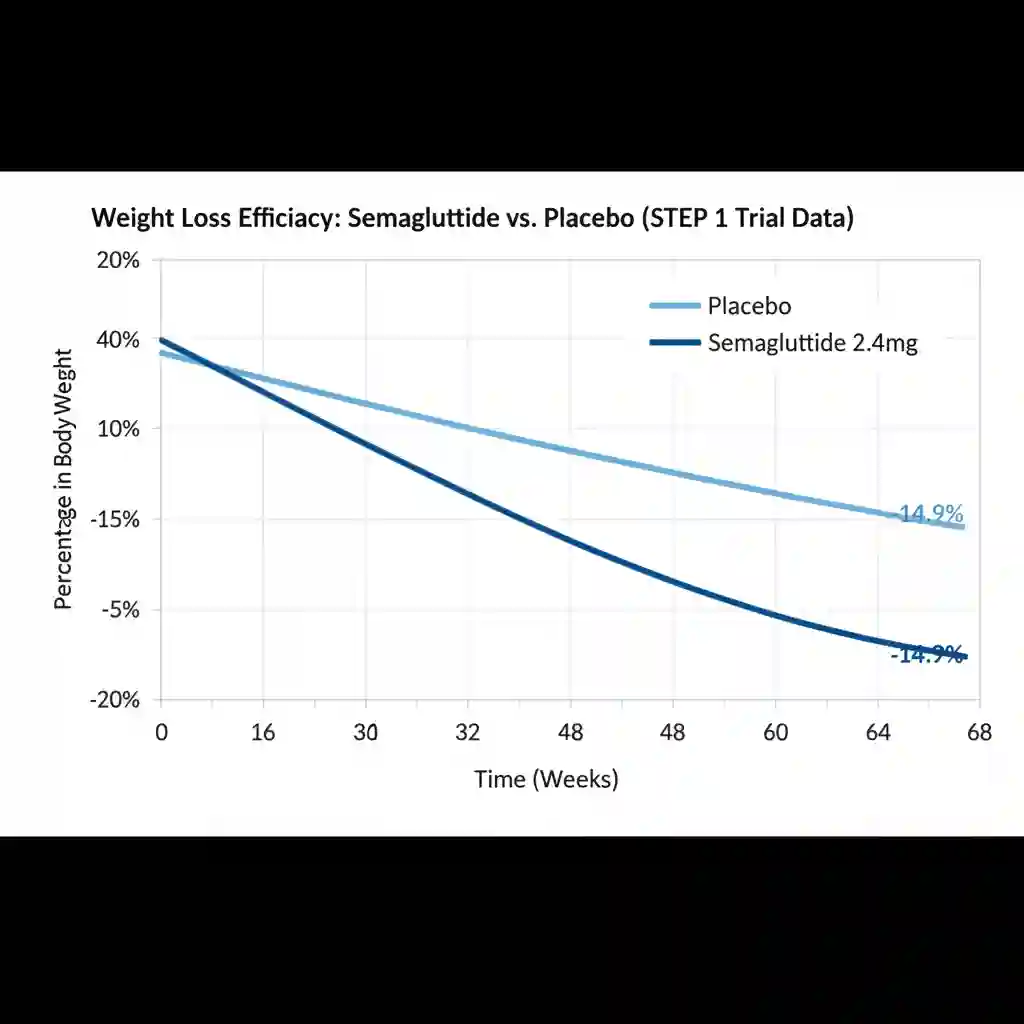
- Efficacy in Weight Reduction: In the STEP 1 trial (Semaglutide Treatment Effect in People with obesity), participants receiving 2.4 mg of semaglutide achieved a mean weight loss of 14.9% over 68 weeks, compared to 2.4% in the placebo group.
- Dual Agonist Potency: The SURMOUNT-1 trial investigating tirzepatide (a dual GIP/GLP-1 agonist) demonstrated even greater efficacy, with the highest dosage group achieving a mean weight reduction of 20.9% over 72 weeks.
- Cardiovascular Benefit: The SELECT trial findings indicated that semaglutide 2.4 mg reduced the risk of major adverse cardiovascular events (MACE)—including cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke—by 20% in adults with overweight or obesity and established cardiovascular disease, independent of weight loss magnitude.
- Metabolic Markers: Treatment is consistently associated with reductions in waist circumference, blood pressure, lipid levels, and inflammatory markers (C-reactive protein).
Research Methodology / Context
Data supporting this trend is primarily derived from large-scale, multicenter, double-blind, randomized controlled trials (RCTs) published in high-impact journals such as The New England Journal of Medicine. These studies typically involve thousands of participants across diverse demographics. The scientific context rests on the physiology of the “incretin effect,” where gut hormones signal satiety to the hypothalamus. Research is now pivoting to long-term observational studies to assess the durability of weight loss, the phenomenon of weight regain upon cessation, and potential long-term adverse effects such as gastroparesis or muscle mass loss (sarcopenia).
Clinical Implications
- Shift in Treatment Algorithms: Clinical guidelines are being updated to recommend GLP-1 agonists as a second-line treatment for obesity (after lifestyle intervention) but often before bariatric surgery, depending on BMI and comorbidities.
- Management of Side Effects: Clinicians must manage common gastrointestinal adverse events (nausea, vomiting, diarrhea) through dose titration. There is an increased need for monitoring gallbladder disease and potential pancreatitis risks.
- Holistic Care Requirement: Due to the risk of lean muscle mass loss alongside fat loss, medical nutrition therapy (MNT) emphasizing protein intake and resistance training is becoming a critical adjunct to pharmacotherapy.
- Healthcare Economics and Access: The high cost of these biologic agents presents significant challenges for insurance coverage and healthcare equity, necessitating rigorous cost-benefit analyses regarding long-term disease prevention (e.g., preventing progression to diabetes).