Executive Summary
Chimeric Antigen Receptor (CAR) T-cell therapy represents a paradigm shift in the treatment of refractory or relapsed hematologic malignancies. This form of adoptive cell transfer (ACT) involves the genetic engineering of autologous T-cells to express synthetic receptors that target specific tumor-associated antigens, independent of Major Histocompatibility Complex (MHC) presentation. Current FDA-approved therapies primarily target the CD19 antigen in B-cell lymphomas and leukemias, and B-cell maturation antigen (BCMA) in multiple myeloma. Research focus is now expanding towards solid tumor applications and allogeneic (off-the-shelf) platforms.
Key Data Points
- Efficacy in DLBCL: Pivotal trials (e.g., ZUMA-1) have demonstrated overall response rates (ORR) of approximately 83%, with complete remission (CR) rates reaching 58% in patients with refractory Large B-Cell Lymphoma.
- Long-term Durability: Follow-up data indicates durable responses, with approximately 35-40% of patients maintaining remission at 5 years post-infusion.
- Toxicity Profile: Cytokine Release Syndrome (CRS) occurs in 30-95% of patients depending on the product, necessitating rigorous management protocols involving Tocilizumab (IL-6 receptor antagonist) and corticosteroids.
- Neurological Events: Immune effector cell-associated neurotoxicity syndrome (ICANS) is reported in 20-60% of cases, varying by costimulatory domain (CD28 vs. 4-1BB).
Research Methodology & Technical Context
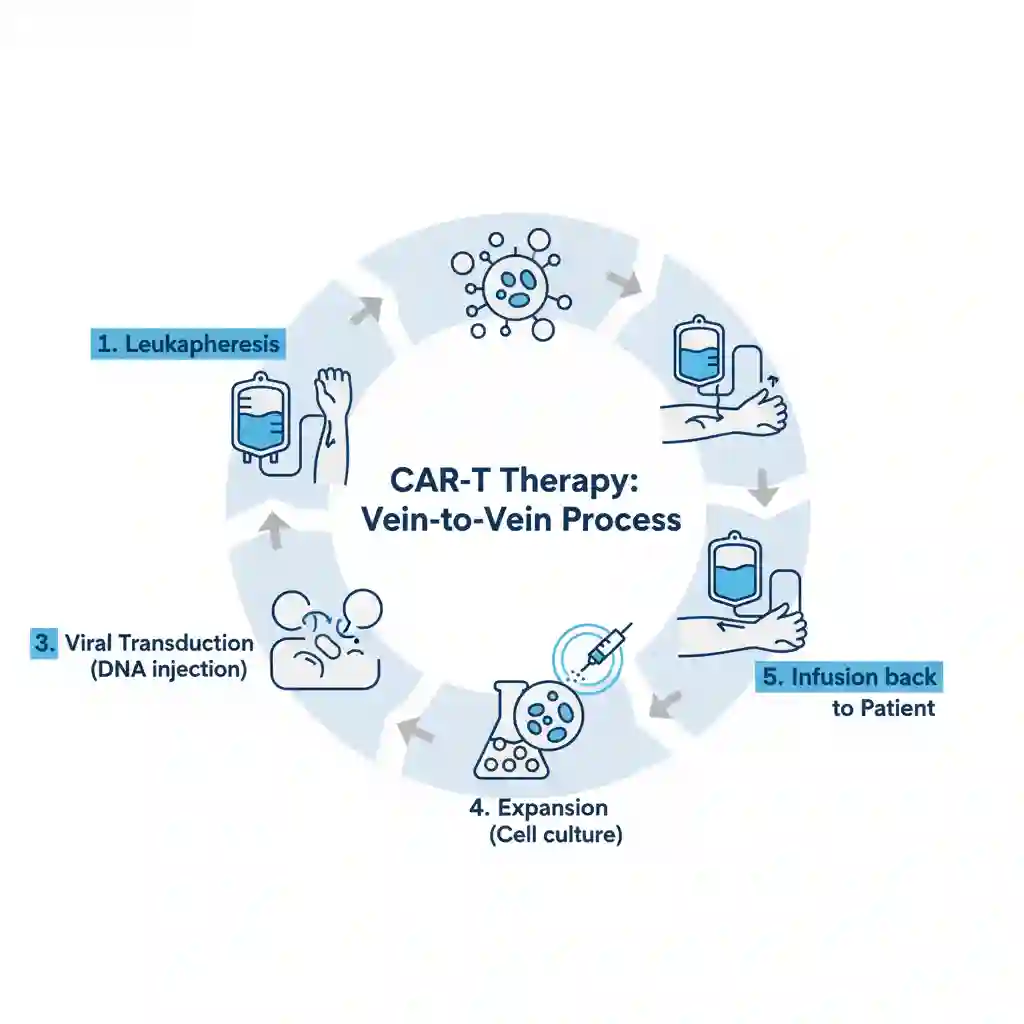
The clinical data is derived principally from multi-center, single-arm Phase II registrational trials and subsequent real-world evidence (RWE) studies. The manufacturing and administration process constitutes a complex logistical chain:
- Leukapheresis: Collection of patient peripheral blood mononuclear cells (PBMCs).
- Genetic Modification: Ex vivo transduction of T-cells using viral vectors (lentiviral or retroviral) to encode the CAR construct.
- Ex Vivo Expansion: Culturing cells to reach a clinically therapeutic dose (typically 10^6 to 10^8 cells/kg).
- Lymphodepletion: Pre-conditioning chemotherapy (typically Fludarabine/Cyclophosphamide) administered to the patient to enhance CAR-T engraftment prior to infusion.
Clinical Implications
The integration of CAR-T therapy into standard oncology practice necessitates significant structural changes in healthcare delivery:
- Infrastructure Requirements: Hospitals must establish FACT-accredited cellular therapy units capable of managing high-grade immunotoxicities (CRS/ICANS).
- Economic Impact: With treatment costs exceeding $370,000 per dose (excluding hospitalization), there is an urgent need for value-based reimbursement models and outcome-based pricing agreements.
- Future Development: Research is shifting towards “Armored CARs” (secreting pro-inflammatory cytokines) to overcome the immunosuppressive tumor microenvironment (TME) in solid tumors.