Quick Facts Summary
| Key Parameter | Pharmacological Data |
|---|---|
| Pharmacologic Class | Angiotensin-Converting Enzyme (ACE) Inhibitor |
| ATC Code | C09AA03 |
| Primary Mechanism | Competitive inhibition of ACE, reducing Angiotensin II synthesis. |
| Key Distinguisher | Not a prodrug; not metabolized by the liver. |
| Black Box Warning | Fetal Toxicity (Pregnancy Category D) |
Detailed Clinical Profile
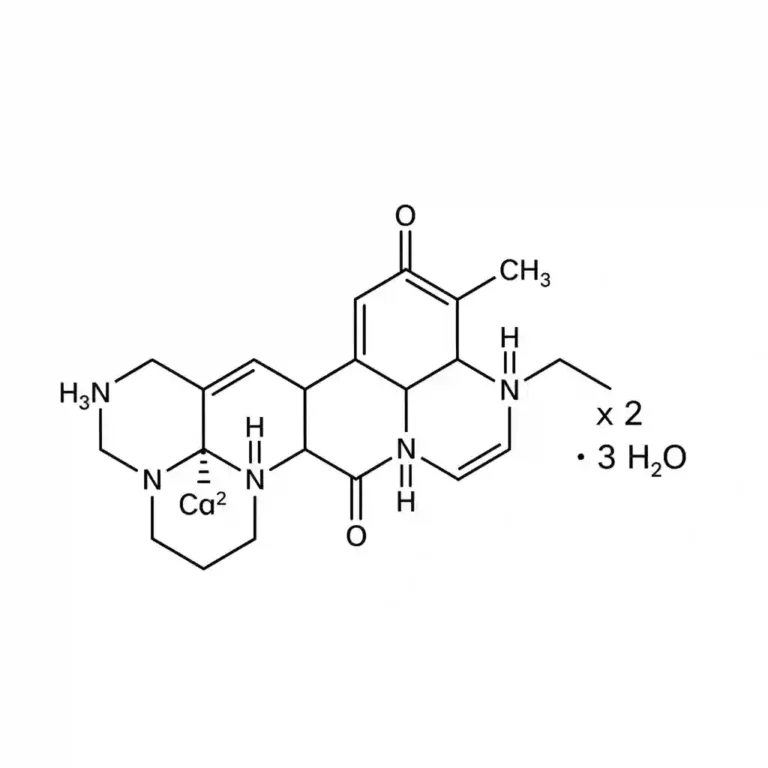
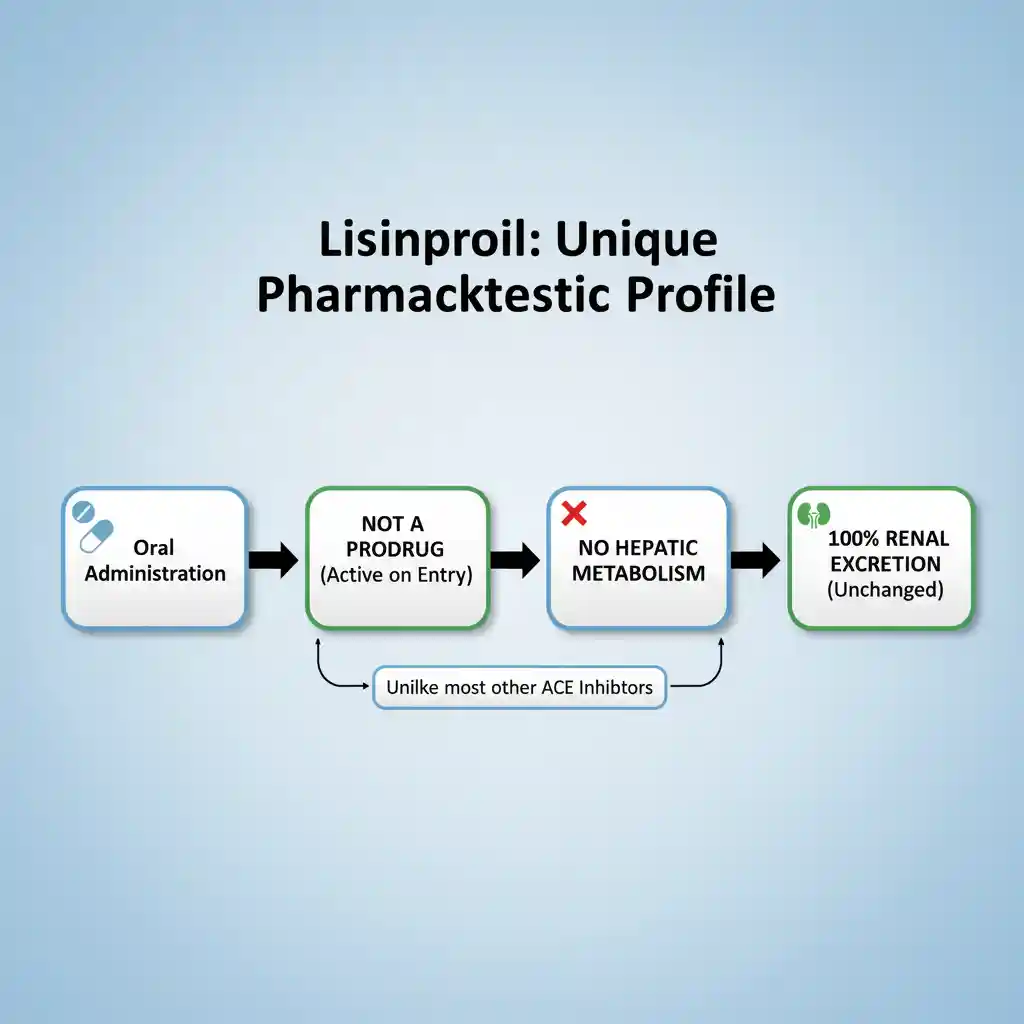
Lisinopril is a synthetic peptide derivative and a long-acting, competitive inhibitor of the angiotensin-converting enzyme (ACE). Unlike many other agents in this class (such as enalapril or ramipril), lisinopril is a lysine analogue of enalaprilat and is not a prodrug. It is pharmacologically active upon administration and does not require hepatic bioactivation. It is indicated for the management of hypertension, heart failure with reduced ejection fraction, and acute myocardial infarction. (Common trade names include Zestril and Prinivil).
Mechanism of Action (MOA)
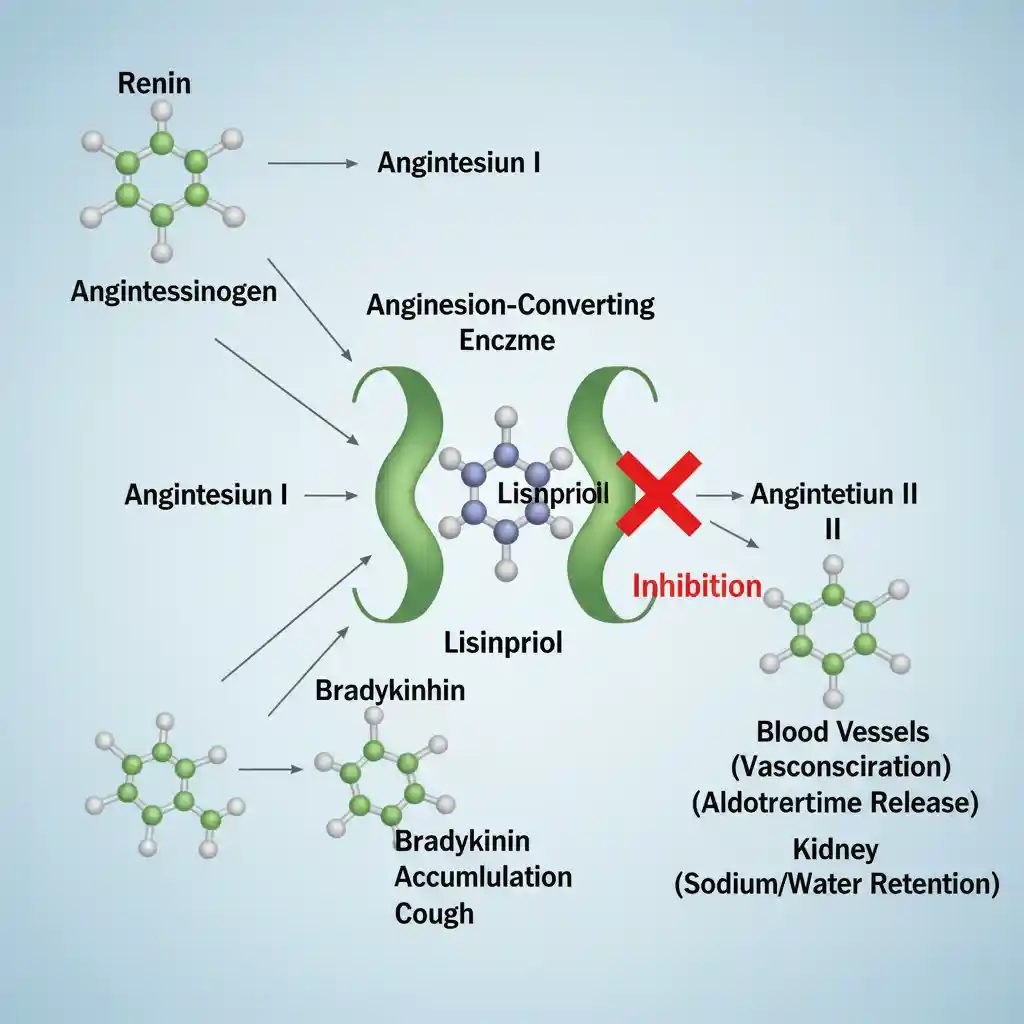
The primary mechanism of action involves the suppression of the Renin-Angiotensin-Aldosterone System (RAAS). Lisinopril binds to and inhibits ACE, a peptidyl dipeptidase that catalyzes the conversion of the decapeptide angiotensin I to the octapeptide angiotensin II.
Downstream Effects of ACE Inhibition:
- Reduced Angiotensin II: Since Angiotensin II is a potent vasoconstrictor, its reduction leads to decreased systemic vascular resistance (vasodilation) and reduced afterload. (See also: Hypertension Pathophysiology).
- Decreased Aldosterone Secretion: Lower Angiotensin II levels reduce adrenal secretion of aldosterone, promoting natriuresis (sodium excretion) and potentially leading to potassium retention.
- Inhibition of Bradykinin Degradation: ACE is structurally identical to Kinase II, the enzyme responsible for breaking down bradykinin. Lisinopril inhibits this breakdown, leading to elevated local levels of bradykinin. While bradykinin contributes to vasodilation, its accumulation in the respiratory tract is the primary mechanism for the characteristic dry cough and potentially angioedema associated with ACE inhibitors.
Pharmacokinetics (PK Profile)
Lisinopril exhibits a unique pharmacokinetic profile among ACE inhibitors due to its hydrophilicity and lack of metabolism.
- Absorption: The absolute bioavailability is approximately 25%, but with significant inter-patient variability (6% to 60%). Absorption is not significantly influenced by food.
- Distribution: It does not bind to serum proteins. It crosses the blood-brain barrier poorly.
- Metabolism: Lisinopril is not metabolized by the liver. It circulates entirely as the unchanged drug. This makes it a preferred choice in patients with hepatic impairment compared to ACE inhibitors that are prodrugs requiring hepatic activation.
- Excretion: Eliminated entirely unchanged via the kidneys. The effective half-life of accumulation is 12.6 hours. Renal clearance correlates with creatinine clearance; therefore, dosage adjustments are required in patients with renal impairment.
Clinical Indications (FDA Labeling)
Lisinopril is FDA-approved for the following clinical scenarios:
- Hypertension: Used as monotherapy or in combination with other antihypertensive agents (e.g., thiazide diuretics).
- Heart Failure: Adjunctive therapy in the management of heart failure with reduced ejection fraction (HFrEF) to improve survival. (See also: Heart Failure Guidelines).
- Acute Myocardial Infarction: Administered within 24 hours in hemodynamically stable patients to improve survival.
Contraindications & Black Box Warnings
Black Box Warning: Fetal Toxicity
Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Lisinopril should be discontinued as soon as pregnancy is detected.
Major Contraindications:
- History of angioedema related to previous ACE inhibitor therapy.
- Hereditary or idiopathic angioedema.
- Co-administration with aliskiren in patients with diabetes.
Frequently Asked Questions (Clinical Context)
Why does Lisinopril cause a dry cough?
The dry cough is caused by the accumulation of bradykinin and substance P in the lungs. ACE inhibitors block the enzyme (Kinase II) responsible for breaking down these inflammatory peptides. The cough is typically non-productive, persistent, and resolves only upon discontinuation of the drug.
Is Lisinopril nephrotoxic or renoprotective?
Lisinopril is generally renoprotective in chronic kidney disease (CKD), particularly in diabetic nephropathy, as it reduces intraglomerular pressure and proteinuria. However, in states of renal hypoperfusion (e.g., renal artery stenosis or dehydration), it can precipitate acute kidney injury by reducing the efferent arteriolar constriction needed to maintain GFR.