Executive Summary
GLP-1 (Glucagon-like peptide-1) receptor agonists represent a paradigm shift in the management of type 2 diabetes and obesity. Originally developed as incretin mimetics to improve glycemic control, their significant effects on weight reduction and cardiovascular protection have established them as a cornerstone of metabolic medicine. This class of therapeutics, including agents like semaglutide and liraglutide, has demonstrated robust efficacy in large-scale clinical trials, leading to an expansion of their clinical indications beyond diabetes management to include chronic weight management and cardiovascular risk reduction.
Key Data Points
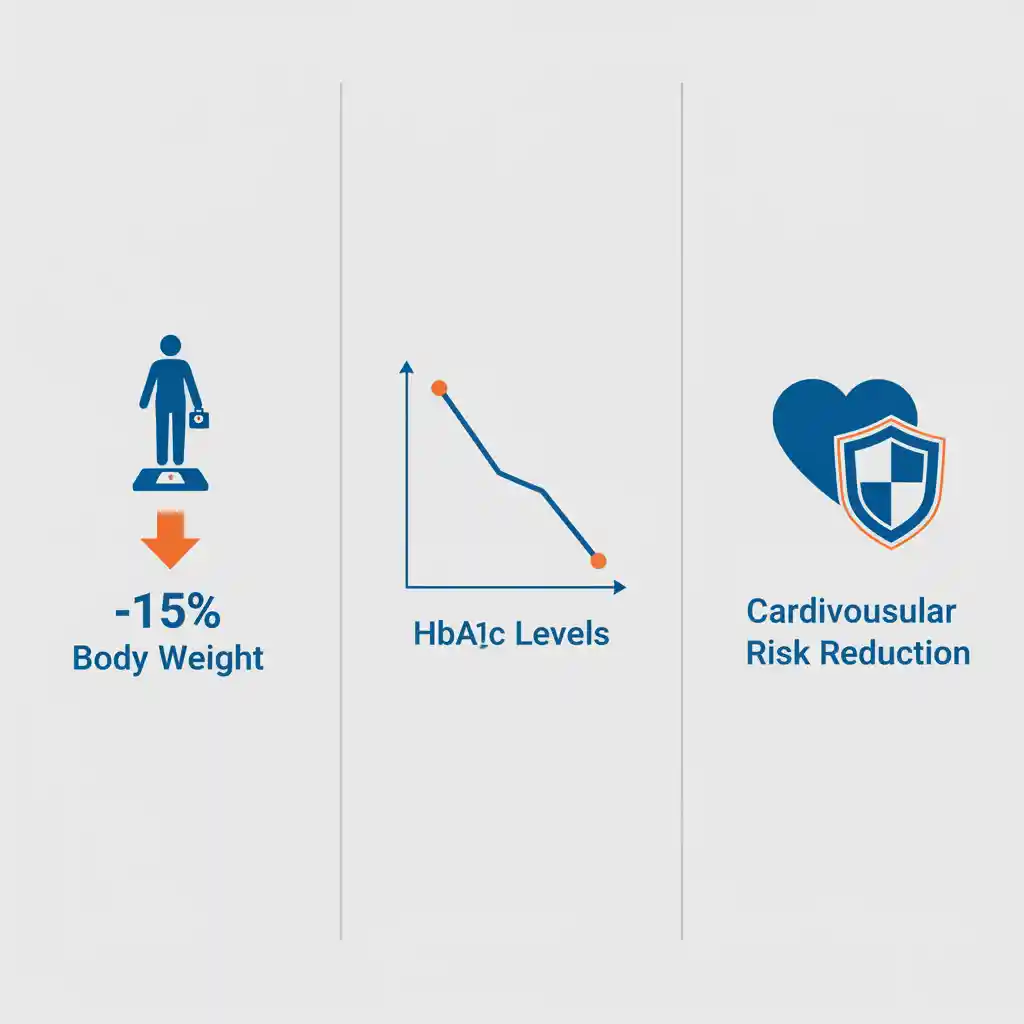
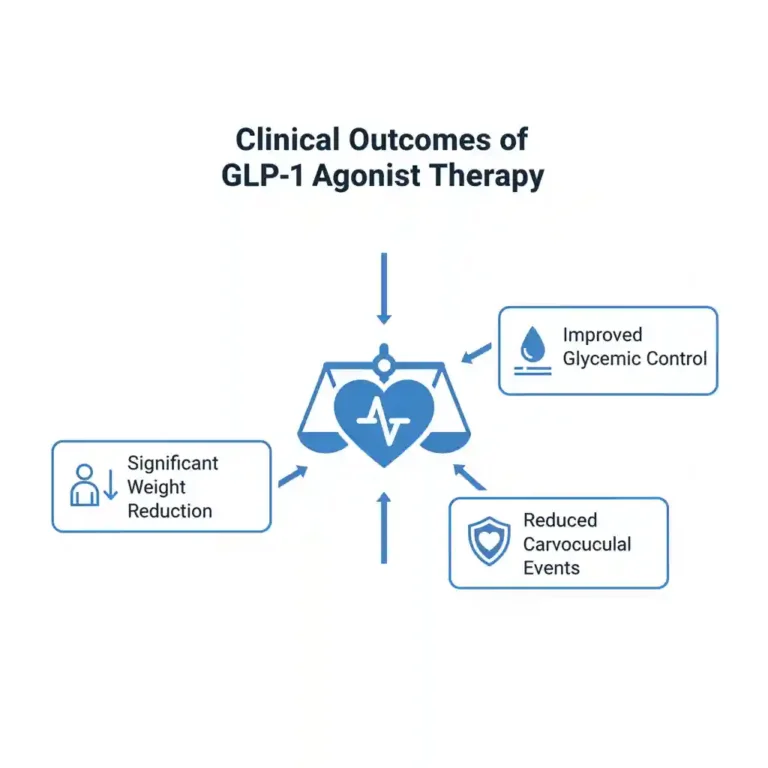
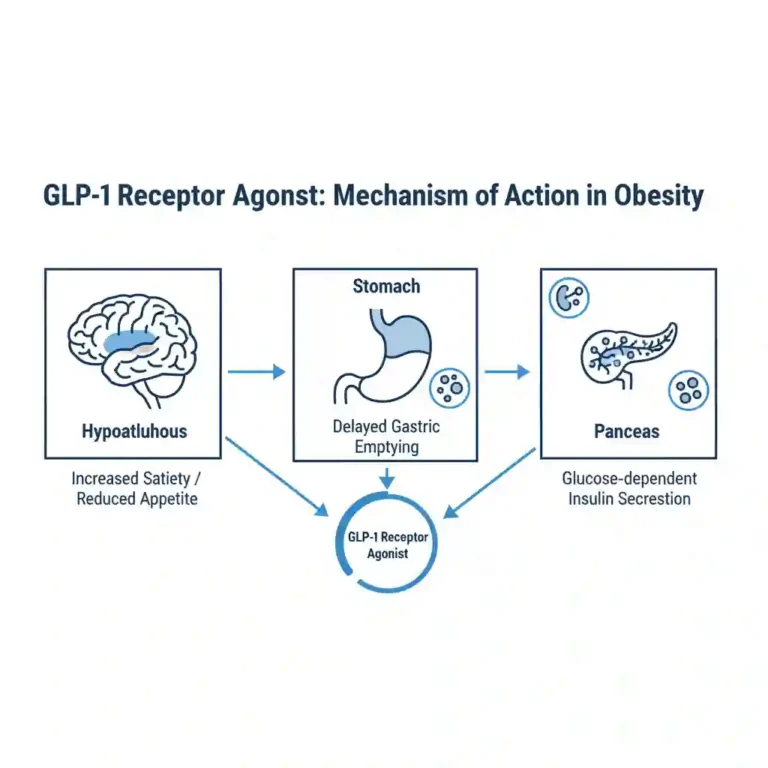
- Glycemic Control: In patients with type 2 diabetes, GLP-1 receptor agonists have been shown in numerous meta-analyses to produce a significant reduction in HbA1c levels, typically ranging from 1.0% to 2.0%, often with a low intrinsic risk of hypoglycemia.
- Substantial Weight Reduction: Pivotal randomized controlled trials (RCTs), such as the STEP program, have demonstrated that higher doses of agents like semaglutide can lead to a mean body weight reduction of 15-20% in individuals with obesity, a level of efficacy that approaches bariatric surgery.
- Cardiovascular Risk Reduction: Cardiovascular Outcome Trials (CVOTs) have established that several GLP-1 receptor agonists significantly reduce the risk of Major Adverse Cardiovascular Events (MACE), including cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke.
- Mechanism of Action: Beyond stimulating insulin secretion, their efficacy is driven by central nervous system effects that increase satiety and reduce appetite, as well as by delaying gastric emptying.
Research Methodology / Context
The evidence for the efficacy and safety of GLP-1 receptor agonists is primarily derived from large-scale, multicenter, double-blind, randomized controlled trials (RCTs). These studies serve as the gold standard for regulatory approval and the formation of clinical guidelines. For diabetes drugs, Cardiovascular Outcome Trials (CVOTs) have become a mandatory research context, specifically designed to assess the cardiovascular safety and potential benefits of new therapies in high-risk populations. This robust methodology has been crucial in validating the multi-faceted benefits of this drug class beyond glucose lowering.
Clinical Implications
-
- Shift in Treatment Paradigm: The clinical implications are profound, marking a shift from a glucocentric approach to a holistic strategy for managing metabolic disease that equally prioritizes weight loss and cardiovascular risk reduction.
–
- New Standard of Care: GLP-1 receptor agonists are now considered a preferred second-line or even first-line therapy for many patients with type 2 diabetes, particularly those with established cardiovascular disease or obesity.
- Expansion of Therapeutic Indications: The official approval of these agents for chronic weight management has opened up a major new therapeutic area in the medical treatment of obesity.
- Future Drug Development: This trend is driving the development of next-generation co-agonists (e.g., GIP/GLP-1) and oral formulations, promising even greater efficacy and patient convenience.