Pharmacological Classification
Class: HMG-CoA Reductase Inhibitor (Statin); Lipid-altering agent.
ATC Code: C10AA05 (Cardiovascular system > Lipid modifying agents > HMG CoA reductase inhibitors)
Common Trade Names: Lipitor (Note: Various generics available).
Mechanism of Action (MOA)
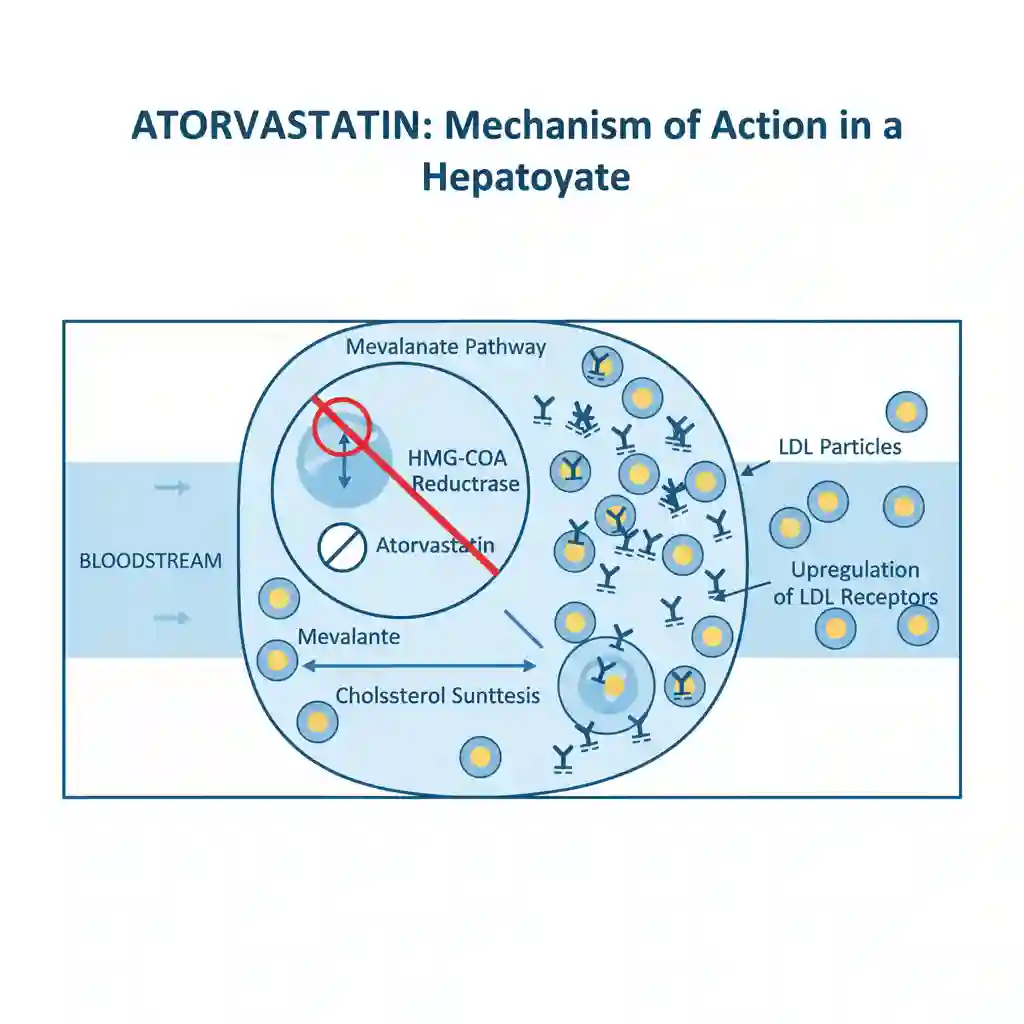
Atorvastatin is a selective, competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme is the rate-limiting catalyst responsible for the conversion of HMG-CoA to mevalonate, a critical precursor of sterols, including cholesterol.
By inhibiting de novo cholesterol synthesis in the liver, atorvastatin triggers a compensatory upregulation of hepatic LDL receptors (LDLR) on the cell surface. This upregulation increases the catabolism and clearance of LDL-cholesterol from the systemic circulation. Additionally, the drug reduces the hepatic synthesis of VLDL-cholesterol and triglycerides.
Pharmacokinetics (PK Profile)
- Absorption: Rapidly absorbed after oral administration; maximum plasma concentrations occur within 1 to 2 hours. However, absolute bioavailability is approximately 14% due to extensive presystemic clearance (first-pass metabolism) in the gastrointestinal mucosa and liver.
- Distribution: Highly protein-bound (≥98%) in plasma. It has a mean volume of distribution of approximately 381 liters. The drug crosses the placenta and is secreted into breast milk (based on animal data).
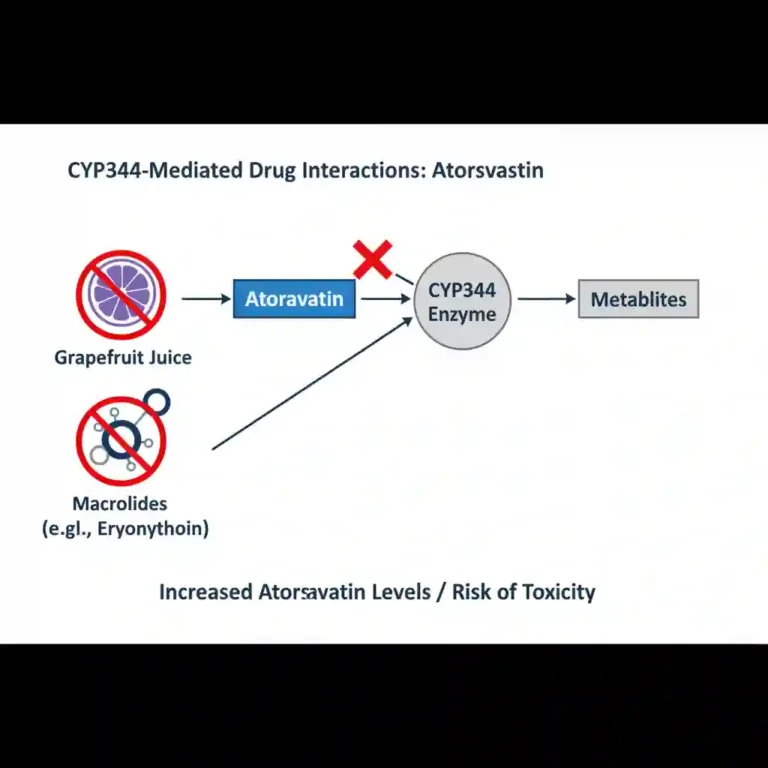
- Metabolism: Extensively metabolized by the hepatic cytochrome P450 system, specifically isoenzyme CYP3A4, to ortho- and parahydroxylated derivatives. Notably, approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites.
- Excretion: Primarily eliminated via hepatic biliary excretion. The mean plasma elimination half-life is approximately 14 hours; however, the half-life of inhibitory activity for HMG-CoA reductase is 20 to 30 hours due to the contribution of active metabolites. Less than 2% is recovered in urine.
Clinical Indications (FDA Labeling)
Atorvastatin is indicated as an adjunct to diet for the treatment of:
- Hyperlipidemia: To reduce elevated total-C, LDL-C, apo B, and TG levels and to increase HDL-C in patients with primary hyperlipidemia (heterozygous familial and nonfamilial) and mixed dyslipidemia.
- Heterozygous Familial Hypercholesterolemia (HeFH): In pediatric patients aged 10 to 17 years.
- Homozygous Familial Hypercholesterolemia (HoFH): To reduce LDL-C and Total-C.
- Cardiovascular Disease Prevention: Prophylaxis in patients with risk factors for coronary heart disease (CHD) to reduce the risk of myocardial infarction, stroke, and revascularization procedures.
Available Preparations: Oral tablets (10 mg, 20 mg, 40 mg, 80 mg).
Contraindications & Safety Warnings
Contraindications: Active liver disease or unexplained persistent elevations of hepatic transaminases; pregnancy and lactation; hypersensitivity to any component of the medication.
Skeletal Muscle Effects: Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported. The risk increases with concurrent administration of strong CYP3A4 inhibitors (e.g., cyclosporine, clarithromycin, itraconazole) or gemfibrozil.
Hepatic Dysfunction: Persistent elevations in serum transaminases (greater than 3 times the upper limit of normal) occurred in 0.7% of patients in clinical trials. Liver enzyme monitoring is recommended prior to initiation.