Biochemical Profile
Classification: Fat-soluble Secosteroid Hormone
Chemical Structure/Properties: Vitamin D exists primarily in two physiological forms: Ergocalciferol (Vitamin D2) and Cholecalciferol (Vitamin D3). Chemically, they are secosteroids, meaning they possess a steroid nucleus with a broken B-ring. Due to their lipophilic nature, they require dietary lipids and bile salts for optimal micellar solubilization and absorption in the intestinal tract. It functions more akin to a steroid hormone than a traditional vitamin cofactor.
Metabolic Function & Mechanism
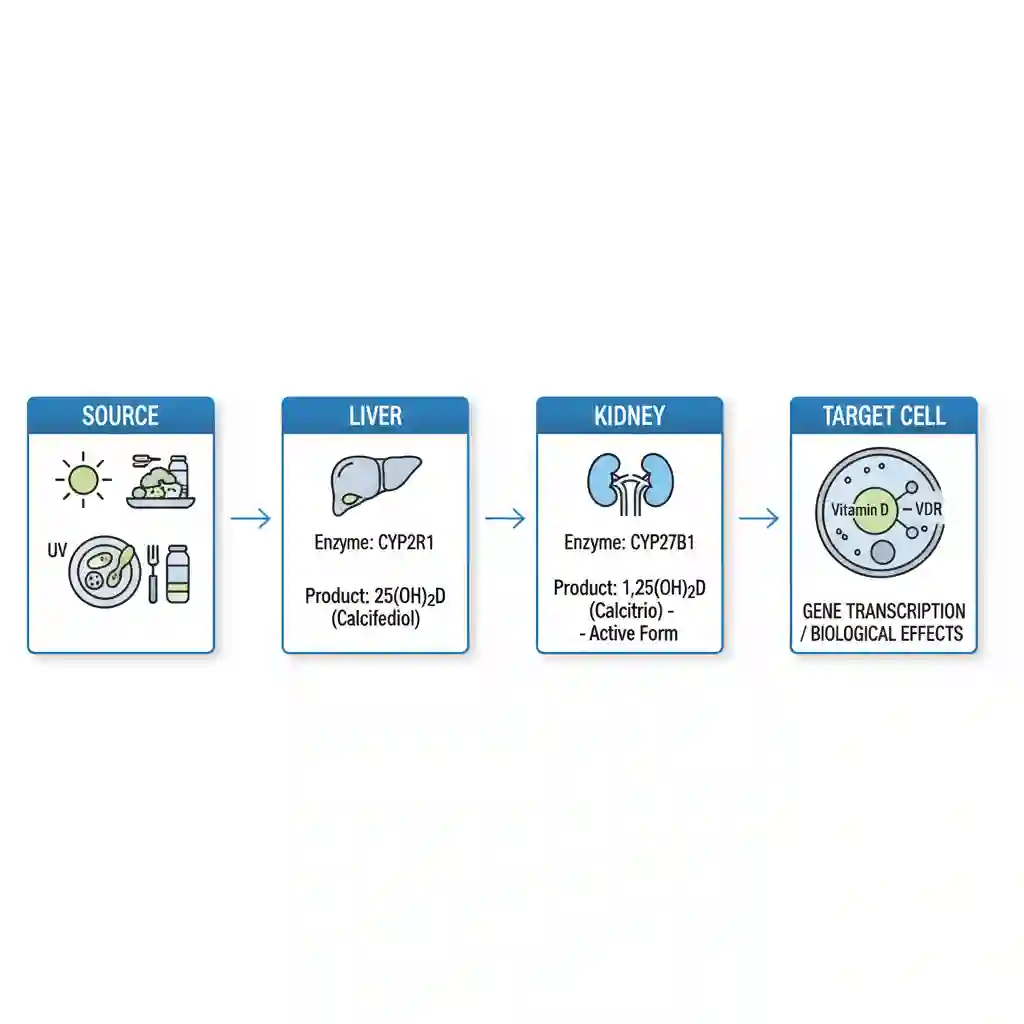
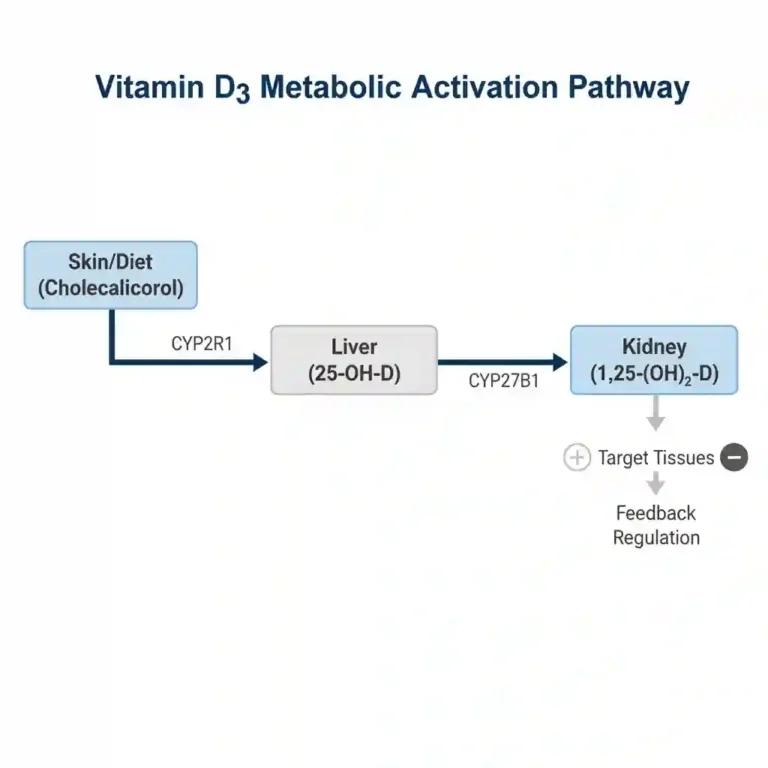
Vitamin D is biologically inert upon ingestion or cutaneous synthesis and must undergo two sequential hydroxylation reactions to become active. First, it is transported to the liver via vitamin D-binding protein (DBP), where it is hydroxylated by 25-hydroxylase (CYP2R1) to form calcifediol (25(OH)D), the major circulating form used for clinical status assessment.
Subsequently, calcifediol is transported to the kidneys, where 1-alpha-hydroxylase (CYP27B1) converts it into the biologically active hormone calcitriol (1,25(OH)2D). At the cellular level, calcitriol binds to the nuclear Vitamin D Receptor (VDR). This complex acts as a transcription factor, regulating the expression of genes involved in calcium and phosphate absorption in the intestine (e.g., TRPV6, calbindin), thereby maintaining mineral homeostasis and skeletal integrity.
Medical Nutrition Therapy (MNT) Applications
Therapeutic supplementation and dietary modification of Vitamin D are indicated in several clinical pathologies:
- Rickets and Osteomalacia: First-line therapy for correcting defective bone mineralization due to severe deficiency.
- Osteoporosis Management: Essential adjunct therapy to optimize calcium absorption and reduce fracture risk in postmenopausal populations.
- Chronic Kidney Disease (CKD – MBD): In CKD stages 3-5, renal conversion to the active form is impaired. MNT often involves the use of active vitamin D analogs (e.g., calcitriol, paricalcitol) rather than cholecalciferol alone to manage secondary hyperparathyroidism.
- Malabsorptive Syndromes: Patients with Cystic Fibrosis, Crohn’s Disease, or post-bariatric surgery require high-dose, monitored supplementation due to impaired lipid absorption.
Dietary Sources & Bioavailability
Bioavailability is significantly influenced by the food matrix, particularly the fat content of the meal.
| Source | Form | Bioavailability Notes |
|---|---|---|
| Fatty Fish (Salmon, Mackerel) | D3 (Cholecalciferol) | High. Naturally encapsulated in lipid matrix, facilitating absorption. |
| Cod Liver Oil | D3 | Very High. Direct lipid delivery vehicle. |
| UV-Exposed Mushrooms | D2 (Ergocalciferol) | Moderate to Low. D2 has a shorter half-life and lower potency in raising serum 25(OH)D compared to D3. |
| Fortified Milk/Plant Milks | D2 or D3 | Variable. Absorption depends on the fat content of the beverage. |
Safety & Interactions
While toxicity is rare, it can lead to hypercalcemia, hypercalciuria, and soft tissue calcification. Clinicians must be aware of significant drug-nutrient interactions:
- Corticosteroids: Chronic use (e.g., prednisone) impairs vitamin D metabolism and calcium absorption, necessitating higher intake requirements.
- Orlistat/Cholestyramine: These agents inhibit fat absorption, thereby reducing the bioavailability of fat-soluble vitamins including D.
- Thiazide Diuretics: Reduce urinary calcium excretion; concurrent high-dose Vitamin D supplementation increases the risk of hypercalcemia.