Pharmacological Classification
Class: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist; Incretin Mimetic.
ATC Code: A10BJ06 (Drugs used in diabetes > Blood glucose lowering drugs, excluding insulins > Glucagon-like peptide-1 (GLP-1) analogues).
Common Trade Names: Ozempic, Wegovy, Rybelsus (Reference only; indications vary by trade name).
Mechanism of Action (MOA)
Semaglutide is a selective GLP-1 receptor agonist with 94% sequence homology to native human GLP-1. It functions by binding to and activating the GLP-1 receptor, a G-protein coupled receptor (GPCR) located on pancreatic beta cells, alpha cells, and neurons in the hypothalamus.
Upon receptor binding, it activates adenylyl cyclase, increasing intracellular cyclic AMP (cAMP) levels. This triggers a signaling cascade that results in:
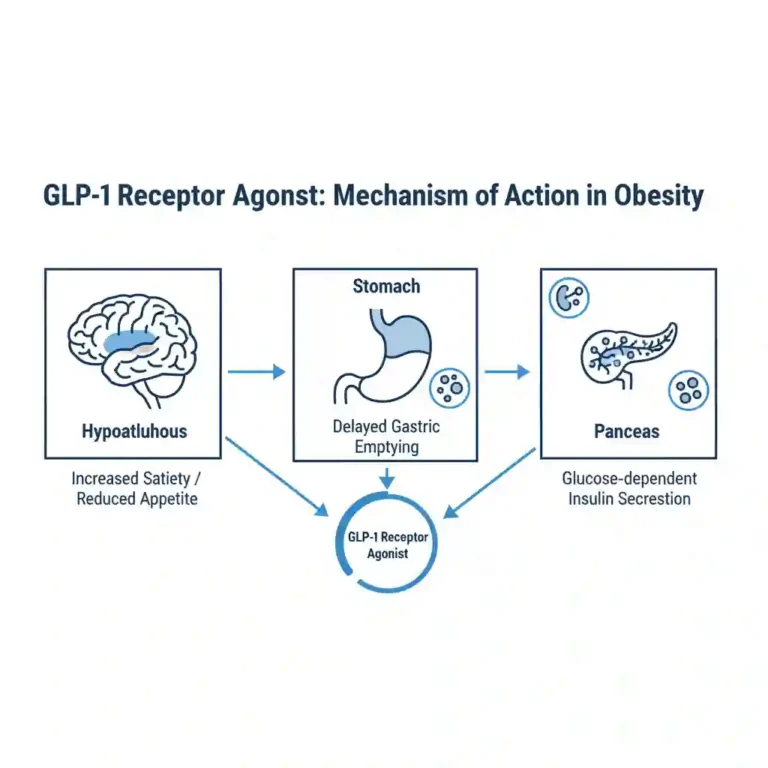
- Glucose-dependent Insulin Secretion: Enhances exocytosis of insulin granules from beta cells only when blood glucose is elevated.
- Glucagon Suppression: Inhibits inappropriate glucagon secretion from alpha cells, reducing hepatic glucose output.
- Gastric Emptying Delay: Slows gastric motility, thereby reducing the rate at which glucose enters the circulation postprandially.
- Central Appetite Regulation: Crosses the blood-brain barrier to interact with the arcuate nucleus in the hypothalamus, promoting satiety and reducing energy intake.
Pharmacokinetics (PK Profile)
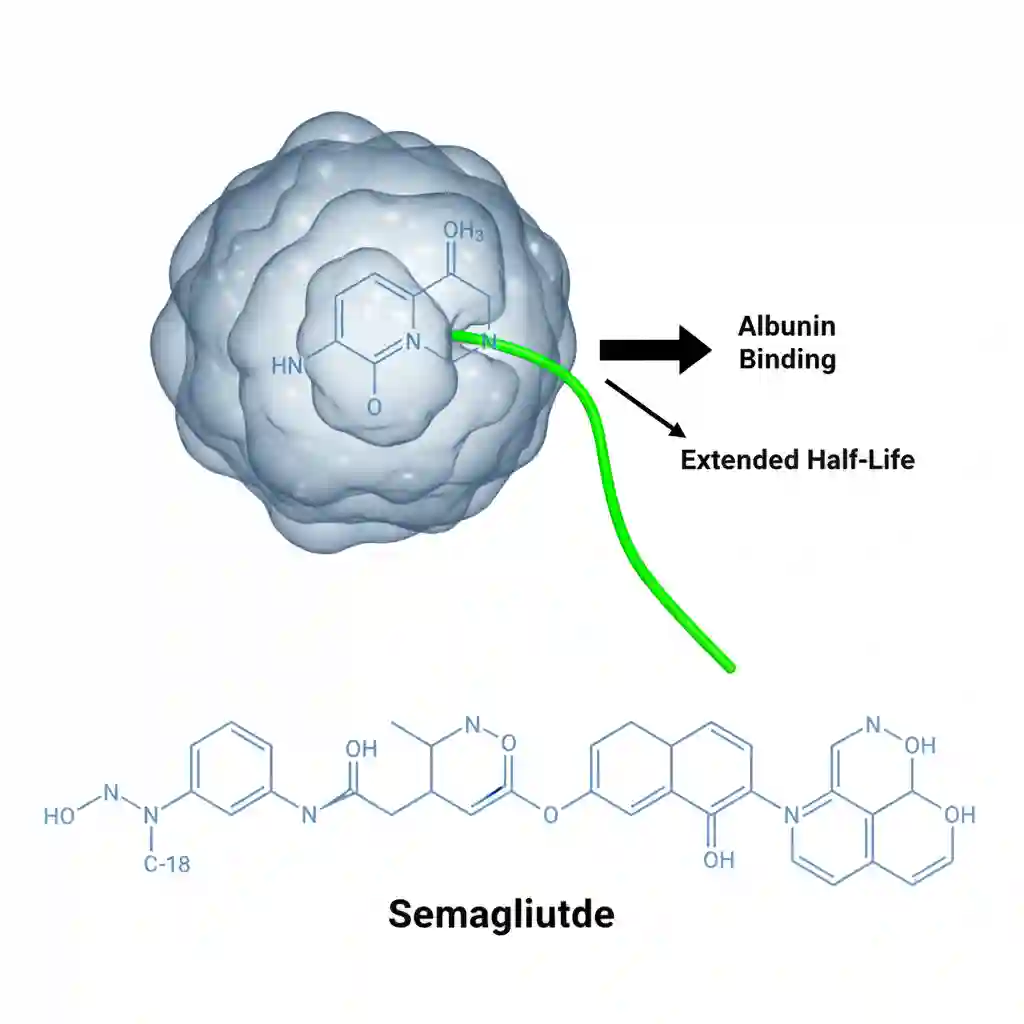
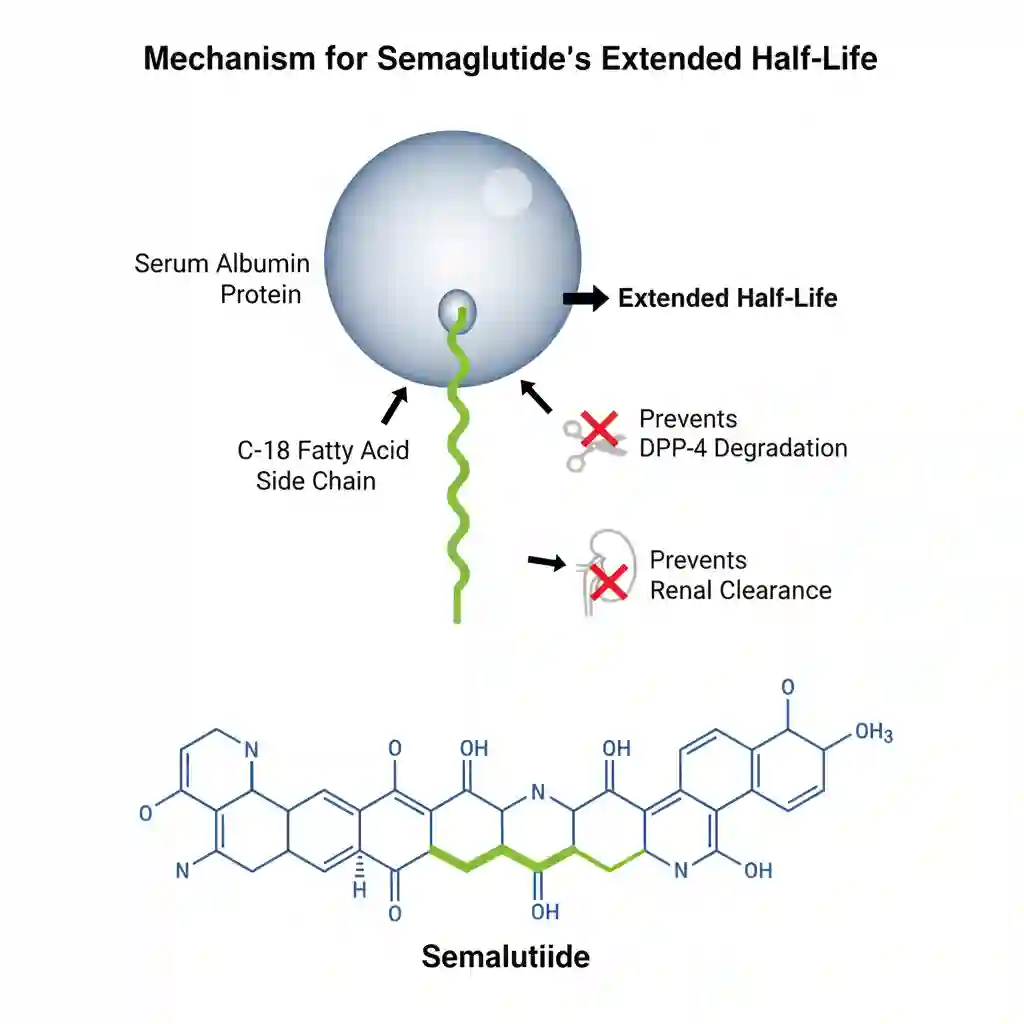
The molecule is structurally modified (acylation with a spacer and C-18 fatty diacid chain) to resist degradation by the enzyme dipeptidyl peptidase-4 (DPP-4) and to bind partially to albumin.
- Absorption:Subcutaneous: Absolute bioavailability is approximately 89%. Maximum plasma concentration (Cmax) is reached 1 to 3 days post-dose.
Oral: Requires co-formulation with SNAC (sodium N-(8-[2-hydroxybenzoyl] amino) caprylate) to facilitate transcellular absorption in the stomach. Bioavailability is low (approx. 0.4-1%).
- Distribution: Extensive plasma protein binding (>99%), primarily to serum albumin. This high binding affinity is the primary driver of its extended half-life. Volume of distribution is approximately 12.5 L.
- Metabolism: Semaglutide is not metabolized by CYP450 enzymes. It undergoes proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid side chain, similar to endogenous protein catabolism.
- Excretion: The primary excretion routes for the degradation products are urine and feces. The elimination half-life is approximately 1 week, allowing for once-weekly administration.
Clinical Indications (FDA Labeling)
Available preparations are indicated for specific metabolic conditions based on the formulation:
- Type 2 Diabetes Mellitus: As an adjunct to diet and exercise to improve glycemic control in adults.
- Cardiovascular Risk Reduction: To reduce the risk of major adverse cardiovascular events (MACE) such as cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke in adults with type 2 diabetes and established cardiovascular disease.
- Chronic Weight Management: Indicated for adults with obesity (BMI ≥30) or overweight (BMI ≥27) in the presence of at least one weight-related comorbid condition (e.g., hypertension, type 2 diabetes, or dyslipidemia).
Contraindications & Black Box Warnings
Safety protocols mandate strict adherence to the following warnings:
- Black Box Warning: Thyroid C-Cell Tumors. In rodent studies, semaglutide caused a dose-dependent and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors. It is contraindicated in patients with a personal or family history of Medullary Thyroid Carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Pancreatitis: Acute pancreatitis has been reported in clinical trials.
- Hypoglycemia: Increased risk when used concomitantly with insulin or insulin secretagogues (e.g., sulfonylureas).
- Acute Kidney Injury: Reports of AKI, sometimes requiring hemodialysis, in patients experiencing severe adverse gastrointestinal reactions (dehydration).